Congrats to Dr. Tan Aidong on Chem. Eng. Sci. publication
文章来源: 发布日期: 2024-04-09
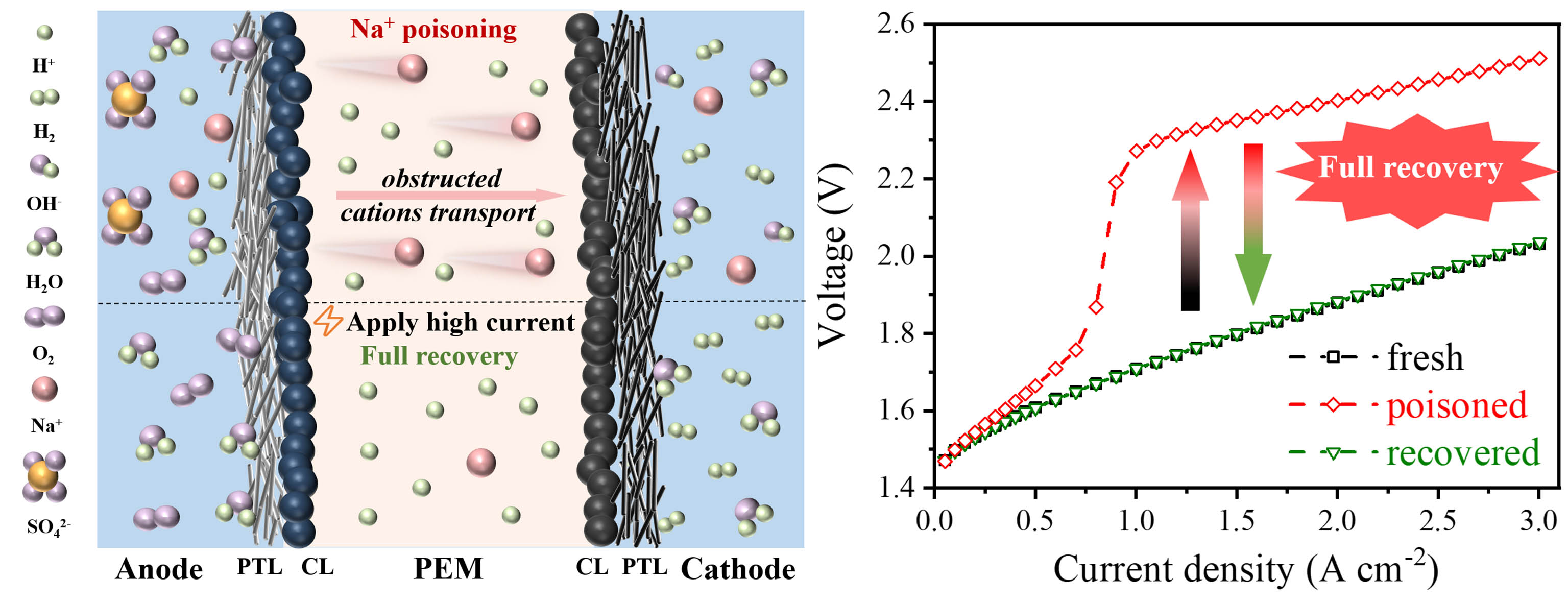
Proton exchange membrane (PEM) water electrolysis represents a feasible technology for ‘green’ hydrogen production. However, the efficiency of the electrolyzers can be compromised by foreign ions in the feed water. In particular, sodium ions (Na+), which are commonly found in water sources, poses a serious threat for the operation of PEM water electrolyzers. This work examines the effects of low-concentration Na+ on the performance of membrane electrode assemblies (MEAs). Results reveal that Na+ can diminish the H+ concentration (cH+) and cation diffusion coefficient (D0) in the isomer and PEM by substituting H+ sites, but doesn’t cause irreversible catalyst or structure damage. The reduced cH+ can lead to a negative shift in equilibrium potential, particularly at the cathode. Concurrently, a lowered D0 slows down the movement of cations, which serve as reactants at the cathode, resulting in impeded mass transport. Additionally, the work presents a methodology for recovering the performance of Na+ poisoned MEAs. Operating the electrolyzer at high current densities, viz. 6.0 A cm-2, could aid in the re-equilibration of Na+/H+ and the expulsion of Na+ from the MEAs. With Pt-coated Ti porous transport layers employed, the poisoned MEAs can be recovered to their original performance levels. This work presents an effective countermeasure to manage the Na+ poisoning in PEM electrolyzers and overcome this prohibitive barrier to the commercial viability of this technology.
https://www.sciencedirect.com/science/article/pii/S000925092400383X